Bronchiolitis obliterans syndrome (BOS) is a manifestation of pulmonary chronic graft versus host disease (cGVHD), a serious complication of allogeneic stem cell transplantation (ASCT). BOS also occurs following bi-orthotopic lung transplant (BOLT), where it is termed chronic lung allograft dysfunction (CLAD)-BOS and is the leading cause of long-term allograft failure. In both contexts, fibrotic obliteration of the small airways leads to progressive respiratory failure. Since the molecular mechanisms leading to fibrotic airway remodeling in humans are poorly understood, we used single cell RNA-sequencing to map intercellular interactions between immune (CD45+ve) and non-immune (CD45-ve) cells of lung explants from patients undergoing first lung transplantation for cGVHD-BOS (n=1) or second lung transplantation for CLAD-BOS (n=2) compared to normal controls (NC) (n=3).
BOS patients ranged in age from 44 to 61 years old; NC were similar in age and died of cerebrovascular accidents. Lungs were surgically removed at the time of BOLT (BOS samples) or at autopsy (NC). CD45+ve and CD45-ve cells were magnetically sorted and freshly processed. Single cell libraries were prepared using the 10X Genomics Chromium Controller and sequenced on an Illumina Novaseq 6000 instrument. Quality control filtering, transcript count normalization, integration, PCA, UMAP projection, and cell clustering were performed with Seurat in R v4.2.1. CD45+ve and CD45-ve cells were annotated in Azimuth using the Human Lung Cell Atlas and LungMAP Cell Cards reference data sets, respectively. Differences in gene expression between conditions were evaluated with the Seurat FindMarkers function. Inter-cellular communications were characterized with CellChat. Interaction strength was calculated as a function of ligand/receptor pair expression, and incoming and outgoing signaling was classified using network centrality analysis.
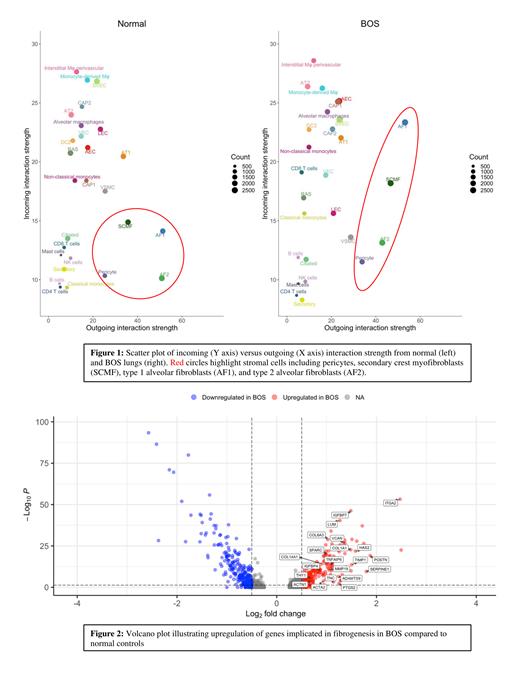
We identified 45,932 CD45+ve cells (28,241 in BOS and 17,691 in NC) and 33,428 CD45-ve cells (23,390 in BOS and 10,038 in NC) including 17 distinct cell types in the CD45+ve fractionand 24 in the CD45-ve fraction. As samples came from patients with end-stage disease, we hypothesized that CD45+ve cells would be highly activated while CD45-ve cells would exhibit strongly fibrogenic programs. To test this hypothesis, we analyzed the incoming and outgoing signal strength for each cell type. Stromal cells such as type 1 (AF1) and type 2 alveolar fibroblasts (AF2), secondary crest myofibroblasts (SCMF), and pericytes had the strongest outgoing signals in BOS ( Figure 1). Except for AF2, incoming and outgoing signaling from stromal cells was stronger in BOS than in NC. These observations support stromal cells as important drivers of BOS pathogenesis.
Next, we characterized signaling directionality between stromal cells and CD45+ve cells. In general, incoming signals to stromal cells from CD45+ve cells were weaker in BOS than in NC, while outgoing signals from stromal cells to CD45+ve cells were stronger. For example, signaling from stromal cells expressing THY1, which may attenuate fibrosis in fibrotic lung disease, to integrins on monocytes and macrophages was stronger in BOS. Likewise, BOS was enriched for signaling from collagens and fibronectin on stromal cells to CD44 on CD45+ve cells.
As expected, genes implicated in fibrosis and wound healing were upregulated in stromal cells in BOS patients. In AF1 ( Figure 2), these included genes encoding collagens (COL6A3, COL14A1), actins (ACTA2), matricellular proteins (POSTN, TNC, SPARC), protease inhibitors (SERPINE1, TIMP1), hyaluronic acid synthetases (HAS2), proteoglycans (LUM, VCAN), and proteases (MMP19, ADAMTS9). Interestingly, we found evidence for active anti-fibrotic programs such as MMP19, PTGS2, and THY1.
To our knowledge, this is the first study employing single cell transcriptomics of lung explants from patients with cGVHD-associated BOS. Robust signaling from stromal cells to CD45+ve cells may support their role in modifying the inflammatory infiltrate in BOS. We identified outgoing, stromal derived signaling associated with fibrotic (collagen and fibronectin) and anti-fibrotic programs (THY1). Our findings illustrate an interplay between pro- and anti-fibrotic programs even in end-stage BOS lungs, raising the tantalizing possibility to intervene therapeutically in this dyshomeostatic balance.
Disclosures
Gill:Kite Pharma: Consultancy; Carisma Therapeutics: Current equity holder in publicly-traded company, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties: patents, Research Funding; Interius Biotherapeutics: Current equity holder in private company, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees, Research Funding; Asher: Research Funding; Currus: Membership on an entity's Board of Directors or advisory committees; Inndura: Membership on an entity's Board of Directors or advisory committees; Mission Bio: Membership on an entity's Board of Directors or advisory committees; NKILT: Membership on an entity's Board of Directors or advisory committees; Vor Bio: Membership on an entity's Board of Directors or advisory committees, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal